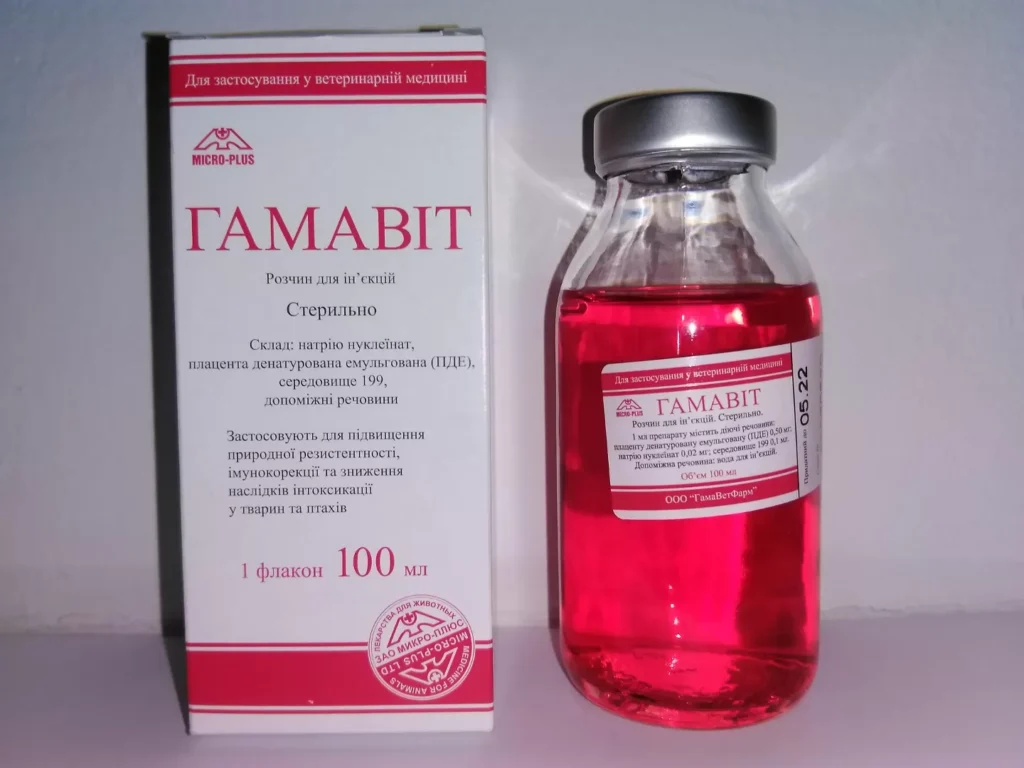
Gamavit Solution For Injection
Gamavit solution for injections is applied to prevention and treatment of various diseases as biotonic: at anemias, hypovitaminoses, at infectious and invasive diseases, a piometra, at poisonings, during the postoperative period, at pregnancy toxicoses, childbirth, when training animals for exhibitions, competitions and transportation.
Gamavit Composition and form of release
Gamavit – a complex drug, the main active ingredients of which are the placenta denatured emulsified (NDE) and sodium nucleinate; the drug is made in liquid form on the basis of a growth nutrient medium containing a balanced solution of salts, amino acids and vitamins. In appearance, the drug is a clear, red liquid. The drug is packaged in 2, 5, 6, 10, 50, 100 or 450 ml in sterile vials of neutral glass, sealed with rubber stoppers and rolled with aluminum caps. Other packing, agreed in the established order is allowed.
Gamavit Pharmacological Properties
Gamavit contains a complex of biologically active substances, which optimizes metabolic processes in the body (in particular, protein, vitamin and mineral), normalizes the blood formula, increases the bactericidal activity of serum, has an immunomodulatory and general biotonizing effect. Is a biogenic stimulant and adaptogen, reduces postnatal mortality, increases the viability of offspring, increases muscle performance and resistance of animals to increased loads and stress. Is a source of metabolic substrates, promotes growth.
Gamavit Injection Indication
The drug is used for the prevention and treatment of various diseases as a biotonizing agent: for anemia, hypovitaminosis, infectious and invasive diseases, pyometra, poisoning, postoperative period, toxicosis of pregnancy, childbirth, in preparation of animals for exhibitions, competitions and transportation.
Doses and method of application:
The drug is administered subcutaneously, intramuscularly, intravenously, possibly drinking.
In order to prevent Gamavit is administered 0.1 ml / kg body weight of the animal (prophylactic dose).
For therapeutic purposes – 0.3-0.5 ml / kg (therapeutic dose).
In order to prevent: anemia, hypovitaminosis, debilitated, old animals, in the recovery period after illness and surgery, the drug is administered intramuscularly 1-3 times a week. The duration of the course averages 2-4 weeks or more. At high loads, to enhance the resistance of animals to technological stress, the drug is administered before stress once or at a rate of 8, 6, 4 days before and immediately before exposure to stressors.
To increase fertility Gamavit Solution For Injection should be administered in a dose of 0.025-0.05 ml / kg on the day of fertilization, to facilitate childbirth and prevent postpartum complications – in the same dose 1 week before delivery and during delivery.
To prevent early mortality, malnutrition, to increase resistance to disease and growth, the drug is administered to newborns on 1, 3 and 5 or 7 days of life at a dose of 0.1 ml / kg
For therapeutic purposes: in the treatment of infectious and invasive diseases (including piroplasmosis) the drug is administered as part of a set of standard therapy 2-3 times a day for 3-5 days. At poisonings Gamavit is entered subcutaneously, intravenously or dropwise 1 time in 3-5 times a therapeutic dose as a part of a complex of means of standard therapy. At feeding disturbances, lag in growth and at intoxications Gamavit is entered 1 time a day within 5-7 days.
At deworming Gamavit is used together with anthelmintics. The drug is administered intramuscularly on the day of deworming and again every other day. In the treatment of obstetric and gynecological diseases Gamavit is used as part of a set of standard therapies once a day for 5-7 days, in pathological births – once in a double therapeutic dose. Gamavit combines well with other pharmacological agents. The combination with Fosprenil enhances the effect of the drug.
Side effects
When using Gamavit Solution For Injection, No side effects or complications have been identified after the use of Gamavit.
Storage conditions
Store the drug at a temperature of 4 ° C to 25 ° C, avoiding freezing. Shelf life – 1 year from the date of manufacture. The drug in vials with impaired integrity, in case of turbidity or discoloration, the presence of impurities, in the expiration date or violation of storage and transportation conditions is not suitable for use.
Read More On Veterinary Supplies
Enhancing Energy For Horses And Riders
Rpm-21 10ml Injections For Camels
Bio Blocker 100ml Injection For Horses And Camels
Buy Dexalone Solution For Camles
Diurizone Horse Anti-inflammatories
Horse Camel Breathing & Endurance Supplement
Anabol 50ml Injection For Horses
Comments
Post a Comment